An atom-thick gold leaf: how is this possible?!
Since ancient times, gold leaf has been used in many fields: in icon painting, architecture, arts and crafts, furniture manufacturing, and others. Gold leaf is made from a small bar of the precious metal.

The production of gold leaf began for the first time in Egypt several thousand years ago. After some time, the technology spread to other countries. Gold leaf comes in various finenesses. But the most common ones are from 22 to 24 carats. The thickness of a gold sheet usually reaches no more than 100 nanometers, that is, several times thinner than paper. It is very light and fragile, as the grains of gold quickly stick together and the sheet can be torn if handled carelessly. Therefore, working with it requires special care.
By the way, if one day in a store you see the food additive E175 on the product’s ingredients list, do not rush to put it back on the shelf. In the food industry, this is how gold leaf is labeled.

Over the centuries, jewelers have sought ways to give gold even more unique forms. But only scientists managed to do this. Modern chemical technology has finally allowed to create a sheet of the precious metal that is literally the thinnest in the world. It consists of only one layer of atoms.
The achievement of Swedish scientists can be compared in significance to the discovery of graphene — a modification of carbon, the thickness of which is also only one atom. The thinnest layer of gold, like graphene, is a good conductor of heat and electricity.
Previously, all attempts to obtain “golden graphene” ended in failure, because the layers of the precious metal stuck together. In addition, the thinnest gold films tend to curl, which made their practical use impossible.

It is worth noting that previously scientists from the University of Leeds have already managed to create one of the thinnest sheets of gold not placed on a substrate of other materials. But it was two atoms thick. They were unable to reach the size of just one atom. But the Swedish scientists were lucky: an accident helped.
Lars Hultman and Shun Kashiwaya, researchers at Linköping University, were working on a completely unrelated project — creating thin films of electrically conductive ceramics based on complex titanium-silicon carbide. A layer of gold was deposited on them, which was supposed to serve as a contact, but under the influence of temperature it gradually replaced the silicon layer in the carbide. Scientists did not expect this, but decided to take advantage of the opportunity to work with the new compound.
Previously, attempts to remove a two-dimensional sheet of metal from the base layer, as is done with ordinary gold leaf, resulted in the metal “sticking together” into grains. But then luck smiled on the chemists again: Hultman came across a Japanese method for removing carbide inclusions from steel, which scientists decided to test on their titanium-gold carbide film. While trying to remove excess layers of carbon and titanium, they accidentally ended up with completely flat sheets of the pure precious metal, one atom thick, not attached to substrates of other materials.
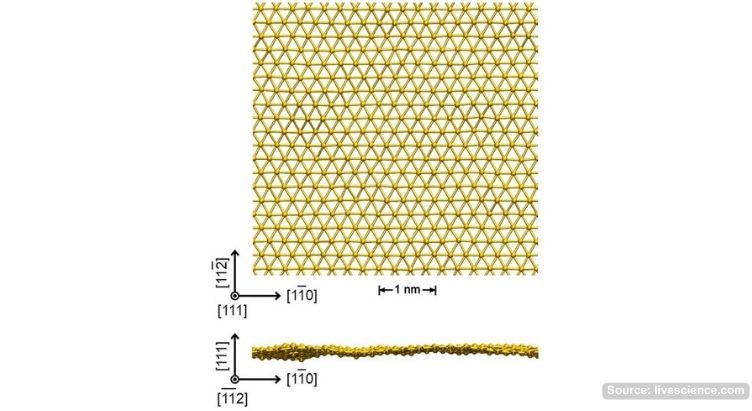
Photo caption: schematic illustration of a sheet of gold just one atom thick
The thinnest sheet of gold can be used in electronics and chemistry. For example, atom-thin sheets of the precious metal can be used to manufacture catalysts that are widely used in medical diagnostics, carbon dioxide conversion, hydrogen production, water purification systems, etc.
Thus, gold is widely used in all walks of life, and its applications are only expanding. Even your phone contains a bit of the precious metal.
Find out where else gold leaf is used in our previous article:
Magnificence of gold: facts on gold leaf